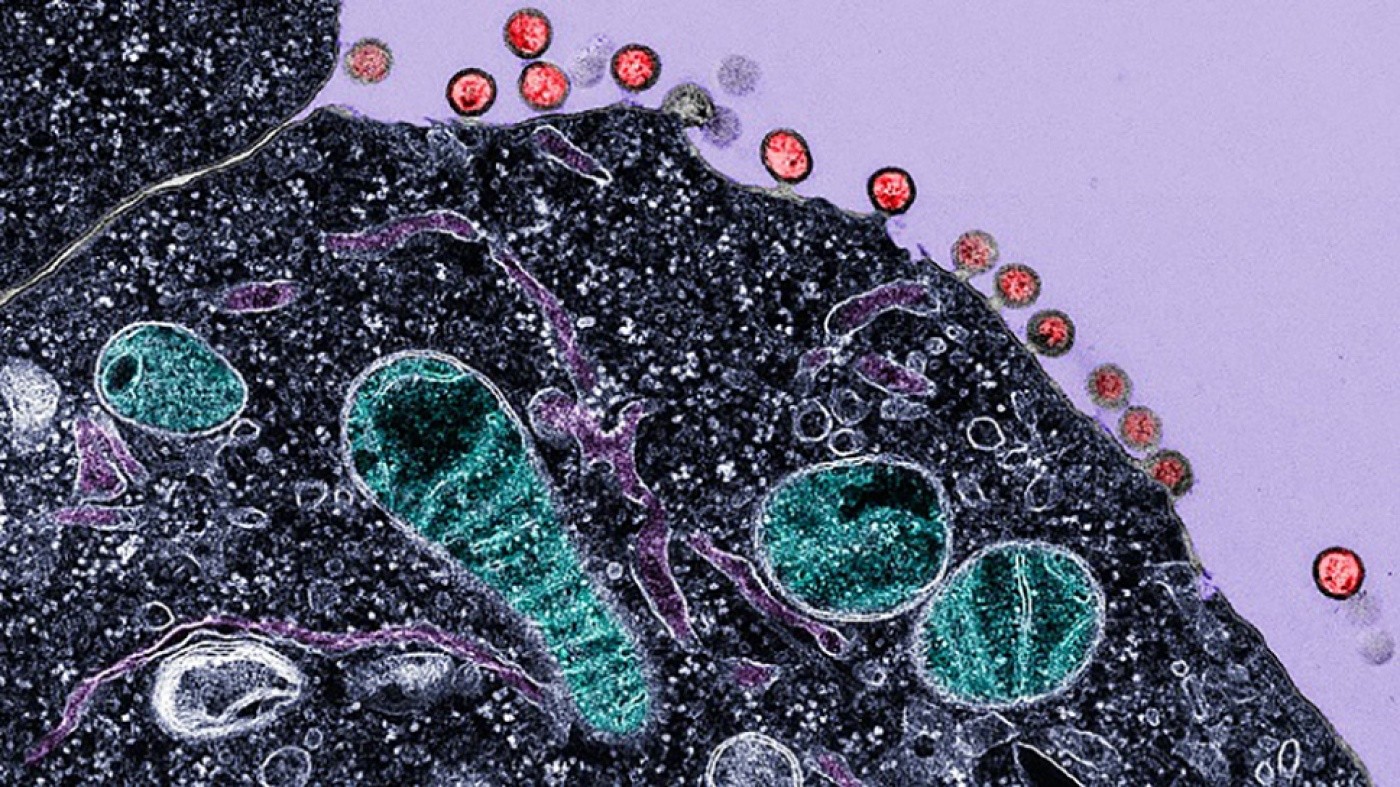
Electron micrograph of cells infected with HIV, demonstrating budding of the virus from the plasma membrane. The red structures are newly synthesized HIV virions budding from the surface of the cell. Mitochondria are labeled in green, intracellular membranes are in dark purple, and the cell cytoplasm is shown in black. [Image: Gilad Doitsh]
On World AIDS Day 2014, people around the globe will consider a number of critical questions about HIV. What progress have we made in curbing the global HIV/AIDS pandemic? Where is HIV research headed, and will we ever have a cure and a vaccine? The answers to many of these questions have long been pursued at the Gladstone Institutes, where investigators are seeking to understand how HIV works, how it hides from the immune system, how it can be killed, and how its transmission from person to person can be blocked.
Past Progress, Present Urgency
Since HIV was first identified as the cause of AIDS in 1984, great strides have been made by Gladstone experts and their collaborators in the fight against this virus. Among the most important developments:
- Dissecting the basics of HIV’s life cycle, which has propelled the development of novel anti-retroviral therapies, converting AIDS from an acute death sentence into a chronic, manageable disease.
- Discovering new ways to prevent HIV infection, including the groundbreaking global iPrEx study that showed how a daily antiretroviral pill can be used to prevent HIV infection in people likely to come in contact with this deadly pathogen.
- Characterizing the proteins in semen that enhance HIV transmission, an understanding that could lead to a new generation of more effective microbicides.
- Identifying a new class of agents (histone deacetylase inhibitors, or HDACs) that might contribute one day to the creation of an HIV cure cocktail.
Despite these and other significant steps forward, over 35 million people worldwide are still infected with HIV, an estimated 2.1 million new cases of AIDS are diagnosed every year, and more than 4,100 people die from the disease each day.
“We have made tremendous progress during the last 30 years in our knowledge of HIV and the creation of drugs to control the disease and keep infected patients alive,” says Warner Greene, MD, PhD, Director of the Gladstone Institute of Virology and Immunology and the Nick and Sue Hellmann Distinguished Professor of Translational Medicine. “But our work is far from done––we still do not have an effective vaccine or a scalable cure.”
 Drs. Warner Greene and Gilad Doitsh are working to find a cure for HIV/AIDS by exploring the basics of viral infection and cellular immune response. [Photo: Chris Goodfellow]
Drs. Warner Greene and Gilad Doitsh are working to find a cure for HIV/AIDS by exploring the basics of viral infection and cellular immune response. [Photo: Chris Goodfellow]
Future Promise: Defining the Cycle of HIV Damage
Dr. Greene, Gilad Doitsh, PhD, and their colleagues at Gladstone have recently published a series of groundbreaking discoveries in the journals Cell, Nature, and Science that have turned the prevailing wisdom about how HIV destroys the immune system on its head—revealing that the body’s cells are not killed by the virus but rather commit suicide in their defense against it.
“These findings have changed our fundamental understanding of how CD4 T cells are lost, and it is the loss of this subset of immune cells that causes AIDS,” explains Dr. Greene. “This opens the possibility of inhibiting the body’s own death-inducing defense as a new way to halt the progression from HIV to AIDS.”
HIV wreaks havoc by attacking the body’s immune system, targeting the “helper” CD4 T cells that regulate immune response. However, the virus is relatively poor at inserting itself into the DNA of these cells, successfully invading just five percent of the ones it attacks. In the other 95 percent—termed bystander cells—the infection is initiated but ultimately blocked by the cells’ defense mechanisms, which protect against full infiltration by the virus’s DNA.
Yet, these bystander cells still die shortly after contact with HIV. Their final demise begins when DNA fragments of the virus remaining inside the cell post-invasion are detected by the sentry protein IFI16, triggering the cell’s alarm system that it is still under attack. This starts a cascade of events, including the release of the inflammatory enzyme caspase 1, which prompts the cell to commit pyroptosis—a particularly fiery form of suicide.
However, instead of protecting against further infection, this action fuels the spread of the virus by attracting new CD4 T cells to the zone of inflammation, perpetuating the cycle of abortive infection and death. Thus, what is meant to serve as a defense against the virus results in the progressive decimation of the body’s immune cells.
New Drugs Block HIV-Induced Cell Suicide
Gladstone scientists identified the underlying mechanisms behind this continual process of cell suicide, and they found a way to stop the deadly cycle: by chemically blocking the release of caspase 1, the cell is unable to combust, thereby cutting the lethal progression short.
Using a class of drugs called caspase 1 inhibitors, one of which has already been shown to be safe and well-tolerated in humans in clinical trials, the investigators have already shown that this strategy works in a dish. Using caspase 1 inhibitors, they were able to prevent lymph cells infected with HIV from killing themselves. Now, research is being conducted to see if the drug has the same effect in a mouse model of HIV as a prelude to human clinical trials.
“With additional fundamental research into how HIV depletes CD4 T cells,” says Dr. Greene, “we are optimistic about finding an improved therapy and potentially new approaches to a cure.”
How Understanding RNA Structure Can Help Researchers Design Better HIV Drugs
How Understanding RNA Structure Can Help Researchers Design Better HIV Drugs
New research sheds light on the biological effect of the many possible three-dimensional structures of a critical loop of RNA in HIV
Research (Publication) HIV/AIDS Virology Ott Lab40 Years of HIV Research: Celebrating the Career of Virologist Warner Greene
40 Years of HIV Research: Celebrating the Career of Virologist Warner Greene
Colleagues look back on Warner Greene's 40 year career
Gladstone Experts History HIV/AIDS Virology Greene LabIn Conversations with Adam Castillejo, the London Patient
In Conversations with Adam Castillejo, the London Patient
Watch this panel discussion that addresses the impact and hurdles associated with developing a cure for HIV
HIV/AIDS Ott Lab